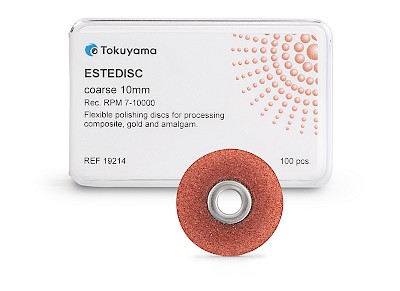
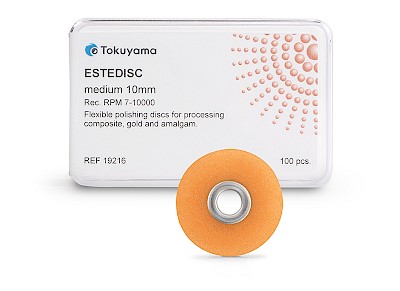
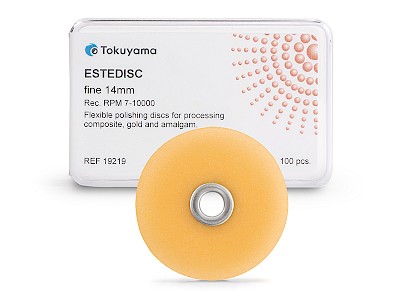
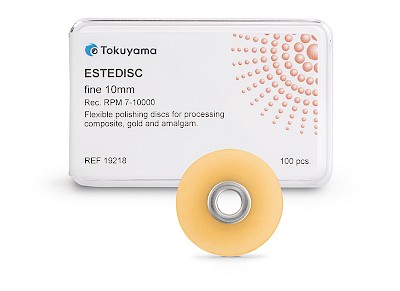
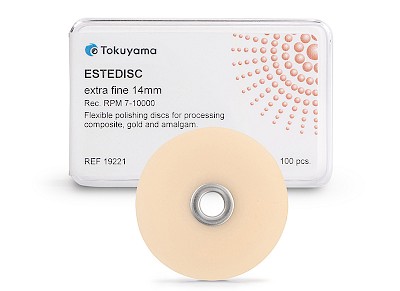
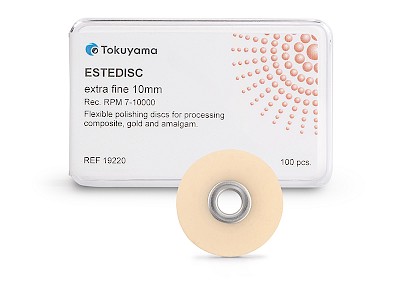
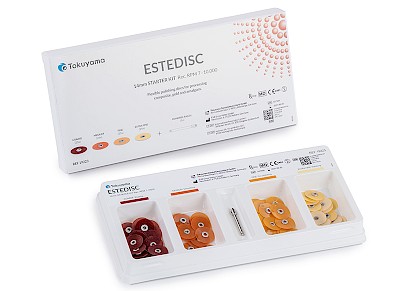
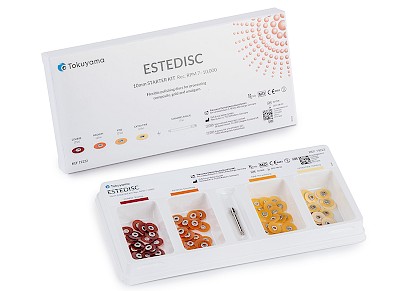
ESTEDISC
Polishing discs for every working step
The flexible ESTEDISC polishing discs from TOKUYAMA DENTAL have been developed for the precise finishing and polishing of composite restorations - from contouring to the final high gloss. Thanks to the aluminium oxide coating on one side, they can be used for both pulling and pushing and enable controlled processing even in areas that are difficult to access.
The different grain sizes allow a step-by-step approach for optimum results with high surface smoothness. ESTEDISC are ideally matched to our composites with spherical fillers and support highly aesthetic, durable and reliable restorations.
Indications
✔ Polishing of composite restorations, gold and amalgam
Features and benefits
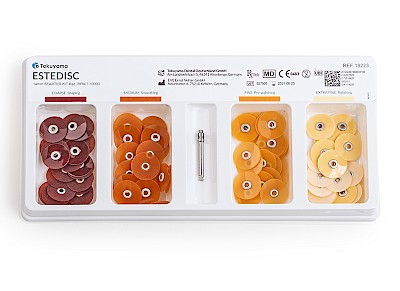
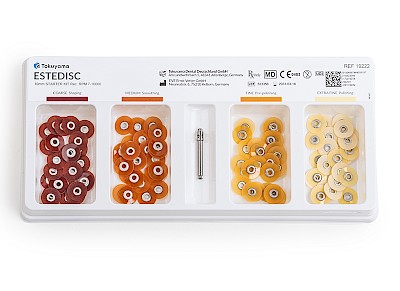
- Flexible polishing system in 4 stages: Coarse (removal) // Medium (smoothing) // Fine (pre-polishing) // Extra fine (polishing)
- Available in two sizes: 10 mm and 14 mm
- For finishing the surface and interproximal polishing
- Coated on one side with aluminium oxide
- Can be used by pulling and pushing
- Precise work even in hard-to-reach areas
Packaging
✔ Single pack: 100 pieces
✔ Starter kit: 4 x 25 pieces + mandrel
Accessory
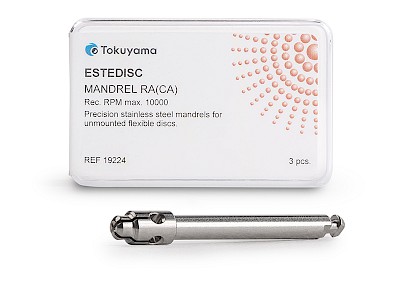
Suitable mandrel for safe application
The ESTEDISC MANDREL RA(CA) is the right holder for all ESTEDISC polishing discs (10 mm and 14 mm) and ensures stable guidance for precise results.
Features and benefits
- Specially developed for ESTEDISC polishing discs
- Secure hold and easy insertion
- Precise guidance when working
- * Our Class IIa devices are certified as a medical device in the European Union under the Medical Device Directive 93/42/EEC by SGS CE1639, exclusively for the indication(s) detailed into the corresponding IFUs. Other non-medical uses ascribed to these devices are not within the scope of CE certification, and users should be aware product performance and/or safety has not been evaluated by SGS for those purposes.